The intersection of AI and drug development has ushered in the transformative era, revolutionizing just how scientists technique biomarker/concentrate on identification, drug/concentrate on interactions, and drug-like molecule style.
Mainly significant for people health care device classes that get in the patient and a lot more critical continue to be there lengthier. Other than that, major subject is leachables from Major packaging substance and after that extractables throughout cleaning method.
Annex 15 of EU-GMP Guideline states that it's as not ample being a SOLE criterion. Visually clean up really should be done Each time possible (at every single cleaning run).
Analytical methods including the Restrict of detection and the limit of quantization of All those methods;
Devices cleaning validation can be done concurrently with true creation measures in the course of procedure development and bulk manufacturing. Validation systems really should be ongoing through full-scale business manufacturing
sixty seven) point out certain types of cleaning validation. Even so, it is normally acknowledged inside the pharmaceutical sector there are two types of sampling methods for cleaning validation: immediate and oblique.
So it is an interaction. For extractables I would like an outlined cleaning procedure and with the materials that perhaps launch extractables I would like to take into account this for cleanability.
· Place again the swab into your tube that contains phosphate buffer and label the tube Together with the screening element and day.
• use distinctive protection click here things for various dosage varieties dependant on physiological reaction (this method is important for powerful resources).
music welcome to a completely new Mastering video clip on Pharmaguideline Within this read more online video We'll know the process with the development of cleaning technique building an effective devices cleaning treatment is vital in the pharmaceutical marketplace to make certain products basic safety high quality and compliance with regulatory criteria Here are a few recommendations to assist you establish a robust devices cleaning process fully grasp equipment and merchandise requirements knowledge of the machines and the specific merchandise It will likely be employed for evaluate the supplies of building product or service compatibility and any specific cleaning problems connected to the equipment seek advice from regulatory guidelines consult regulatory guidelines like People furnished by the FDA or other applicable authorities for cleaning validation specifications and Market ideal techniques ensure that your cleaning procedure aligns with these guidelines to maintain compliance determine vital cleaning measures discover the important cleaning techniques important to rem
In this right after cleaning initially the Visible inspection of kit is finished. When the surface area of apparatus is difficult to examine, a mirror should be used to check cleanliness of equipment.
The results of the plan—along with other excellent and compliance courses such as procedure validation, corrective and preventive action (CAPA), and change Handle techniques—is an important prerequisite of the effectively-instituted high quality management process.
Following the acceptance of any alter based on the procedure, it is needed to revalidate the Cleaning Technique.
7.one Detergents ought to aid the cleaning method and be quickly removable. Detergents which have persistent residues like cationic detergents which adhere pretty strongly to glass and therefore are difficult to clear away, ought to be avoided exactly where feasible.
 Danny Tamberelli Then & Now!
Danny Tamberelli Then & Now! Mara Wilson Then & Now!
Mara Wilson Then & Now!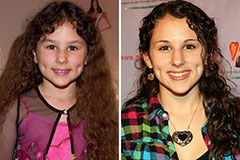 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Michael Bower Then & Now!
Michael Bower Then & Now! Soleil Moon Frye Then & Now!
Soleil Moon Frye Then & Now!